With the launch of ISM5411's first human trial, Insilico Medicine is making significant progress in their quest to tackle IBD.
In Brief
The introduction of ISM5411 marks Insilico Medicine's fifth clinical initiative fueled by AI, specifically targeting the management of inflammatory bowel disease (IBD).

Today, Insilico, a biotechnology company relying on generative AI, has announced the initiation of its first human study for ISM5411. Insilico Medicine ISM5411 is touted as a potentially revolutionary PHD inhibitor for alleviating inflammatory bowel disease (IBD), representing Insilico’s fifth AI-engineered drug to progress to clinical trials, underlining a considerable achievement in their innovative efforts.
Utilizing Insilico's proprietary AI-driven platform for comprehensive drug development, ISM5411 exemplifies the company's pledge to harness advanced technology to tackle intricate medical challenges. drug development initiatives.
According to Alex Zhavoronkov, PhD, the founder and co-CEO of Insilico Medicine, the R&D team employed their proprietary generative AI Chemistry42 to create small molecules based on the character of the PHD target protein. Following the identification of a series of unique lead compounds with distinctive structures, innovative binding characteristics, and impressive efficacy, further refinements in medicinal chemistry were conducted. Pharma.AI After a meticulous selection process, we have designated ISM5411 as an oral inhibitor that specifically targets PHD with a focus on intestinal activity,” Zhavoronkov remarked. medical challenges.
IBD is a chronic inflammatory disorder that impacts the gastrointestinal system and encompasses conditions like ulcerative colitis (UC) and Crohn’s disease (CD). These conditions affect millions worldwide, including around 3 million adults in the United States.
Given that there is currently no cure for IBD, those afflicted face heightened risks of colorectal cancer. The available treatments mainly consist of anti-inflammatory medications, which provide minimal benefits regarding mucosal healing. There is robust clinical and genetic evidence suggesting that effective mucosal healing is closely linked to favorable health outcomes.
The current Phase I trial aims to evaluate the safety, tolerability, pharmacokinetics, and effect of food on ISM5411, with escalating oral doses administered to 76 healthy individuals. The initial dose has successfully been administered to healthy volunteers in Australia, marking a crucial advancement in the drug's developmental pathway.
To broaden the assessment of ISM5411 across diverse populations, Insilico is gearing up for extensive, multi-center Phase Ib trials.
Looking towards the future, Insilico intends to execute an international multi-center clinical study in countries like the United States and China, intending to analyze a wider participant pool following the conclusion of Phase 1a trials. This study will encompass three groups receiving treatment, alongside a placebo group, with specific dosages established based on findings from the Phase Ia study,” remarked Zhavoronkov from Insilico.
Harnessing the power of Generative AI for speeding up drug trial processes is a key part of Insilico’s mission.

The journey of ISM5411 illustrates Insilico's commitment to addressing critical unmet clinical needs, as it was officially recognized as a preclinical candidate back in January 2022.
With its innovative drug development engine, ISM5411 presents a groundbreaking scaffold and a unique binding mechanism. Initial preclinical evaluations have shown strong safety profiles along with remarkable effectiveness against colitis, positioning ISM5411 as a promising small molecule inhibitor specifically targeting the gut.
Research highlights the critical role of Prolyl Hydroxylase (PHD) in maintaining the stability and activity of hypoxia-inducible factor-1α (HIF-1α), which regulates essential barrier-protective gene expressions. PHD levels are significantly elevated in IBD patients and show a strong association with inflammatory markers and apoptosis indicators, suggesting PHD as a viable therapeutic target for treating IBD.
Developed by Chemistry42, Insilico’s generative AI Leading the clinical trial, Dr. Sujata Rao, MD, Chief Medical Officer at Insilico Medicine, emphasizes the potential impact of ISM5411 for patients with limited treatment choices.
“ISM5411 is designed to be an oral drug that specifically targets PHD with a focus on intestinal effects. It serves as an important alternative to both existing therapies and other emerging treatments. One of the standout advantages of our AI-crafted therapy is its ability to promote mucosal repair, which is crucial since IBD currently lacks a definitive cure,” Zhavoronkov noted in an interview with Metaverse Post. “Our preclinical evidence indicates that ISM5411 can both reduce inflammation and enhance mucosal repair, aiming to improve long-term clinical outcomes significantly.”
Insilico Medicine is at the forefront of integrating artificial intelligence with top-tier technology. With over 30 projects in various stages and four currently undergoing clinical trials, Insilico is on a mission to be a trailblazer in the industry.
The company is bolstering its efforts in AI algorithm research and platform development, notably enhancing its capabilities in Canada.
“ISM5411 represents our fifth clinical-stage asset targeting IBD. Notably, our leading drug for idiopathic pulmonary fibrosis was the first to have both a target identified and designed by AI to reach Phase II trials with patient involvement,” mentioned Zhavoronkov. “Moreover, our potentially best-in-class USP1 for BRCA-mutated tumors, which was developed using our platform, has recently been licensed to Exelixis for an upfront fee of $80 million, along with additional milestone payments and royalties.” AI-driven drug discovery .
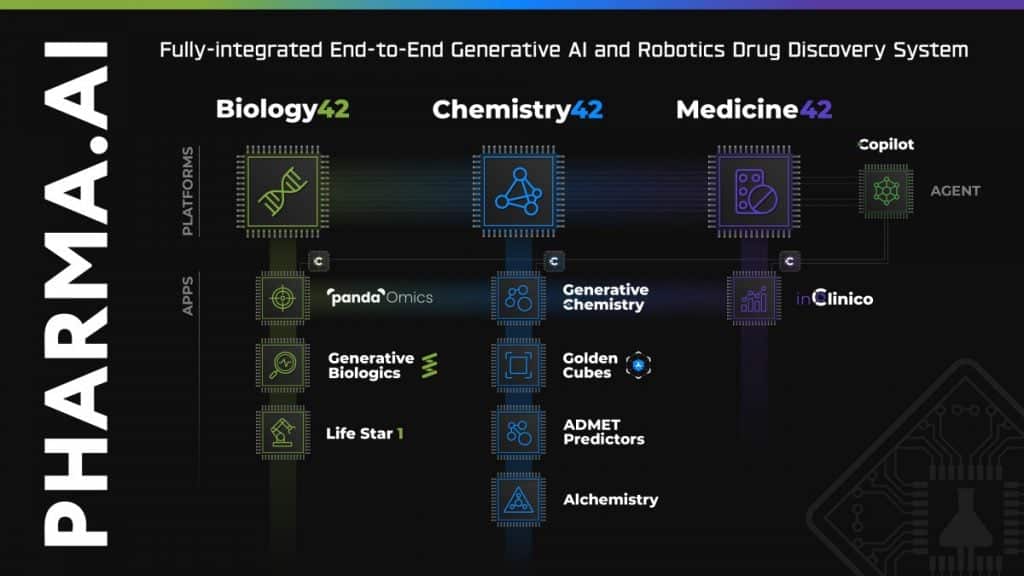
The recent launch of the Al R&D Center in Montreal The Pharma.AI platform received a recent upgrade containing new generative AI functionalities, incorporating chat capabilities for drug discovery, virtual kinase panels for screening off-target activities—dubbed 'Golden Cubes'—and an Alchemistry system to calculate the free binding energy for the created molecules, allowing for comparisons based solely on binding energy, without needing additional physics tools.
Please be aware that the information provided on this page is not intended as legal, financial, or investment advice. It is vital to only invest what you can afford to lose, and we recommend consulting with an independent financial advisor if you have any concerns. For further information, we recommend reviewing the terms and conditions, as well as the support resources provided by your issuer or advertiser. MetaversePost is dedicated to delivering precise and impartial reporting; however, market conditions may change without notice. cancer inhibitor Victor serves as the Managing Tech Editor/Writer at Metaverse Post, focusing on topics such as artificial intelligence, cryptocurrencies, data science, the metaverse, and cybersecurity in the enterprise sector. He has amassed five years of experience in media and AI, having worked for prominent publications like VentureBeat, DatatechVibe, and Analytics India Magazine. As a Media Mentor at prestigious institutions, including Oxford and USC, and holding a Master’s degree in data science and analytics, Victor is committed to staying updated with emerging trends.
He keeps readers informed with the most recent and insightful developments in the Technology and Web3 realms. ChatGPT Cryptocurrencylistings.com’s WCTC S7 is launching, creating a vibrant platform dedicated to trading excellence and collaborative efforts.
Disclaimer
In line with the Trust Project guidelines BitMart is gearing up to showcase its innovations at TOKEN2049 Dubai, marking a pivotal moment in its global influence.







